Electronic Data Capture (EDC)
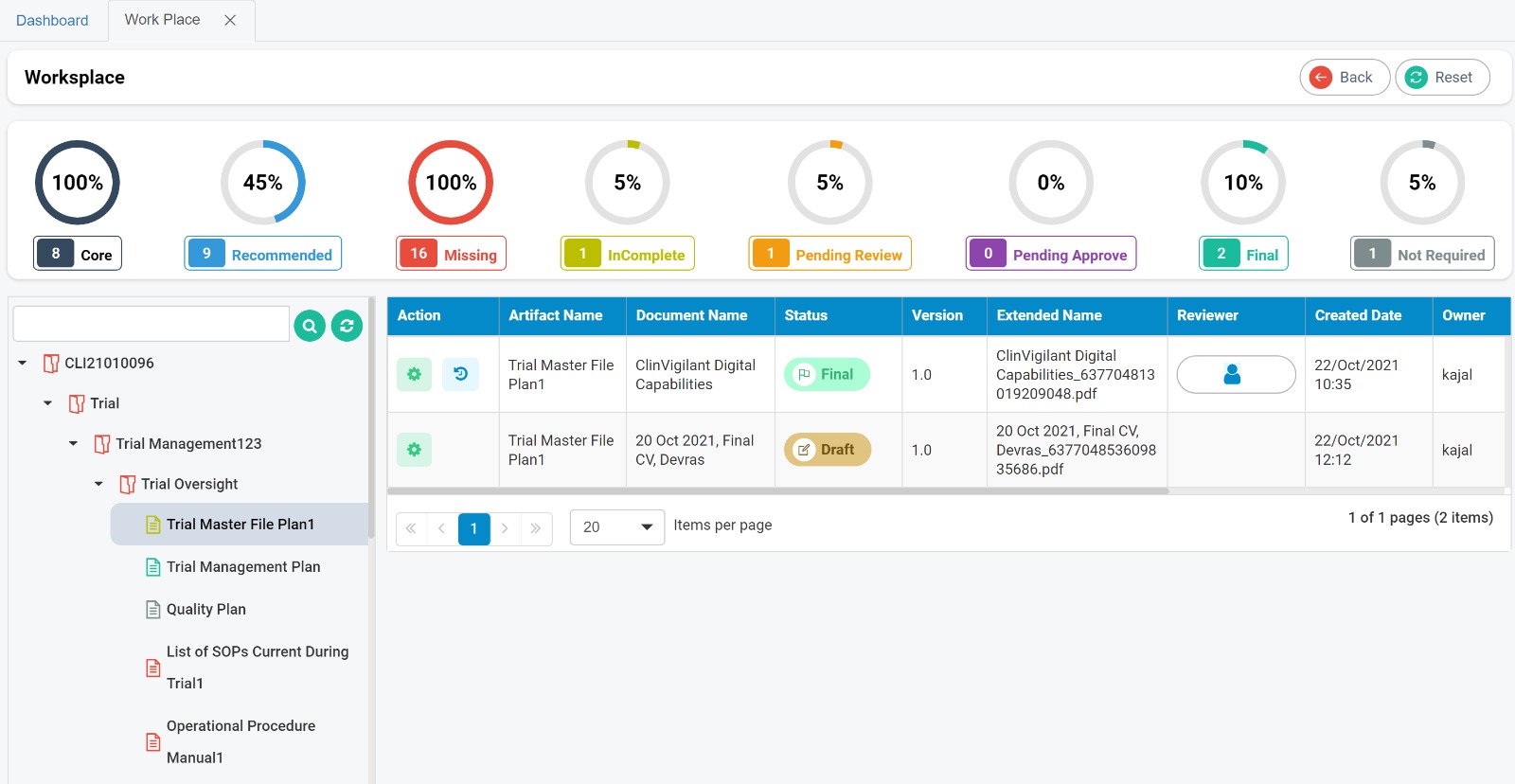
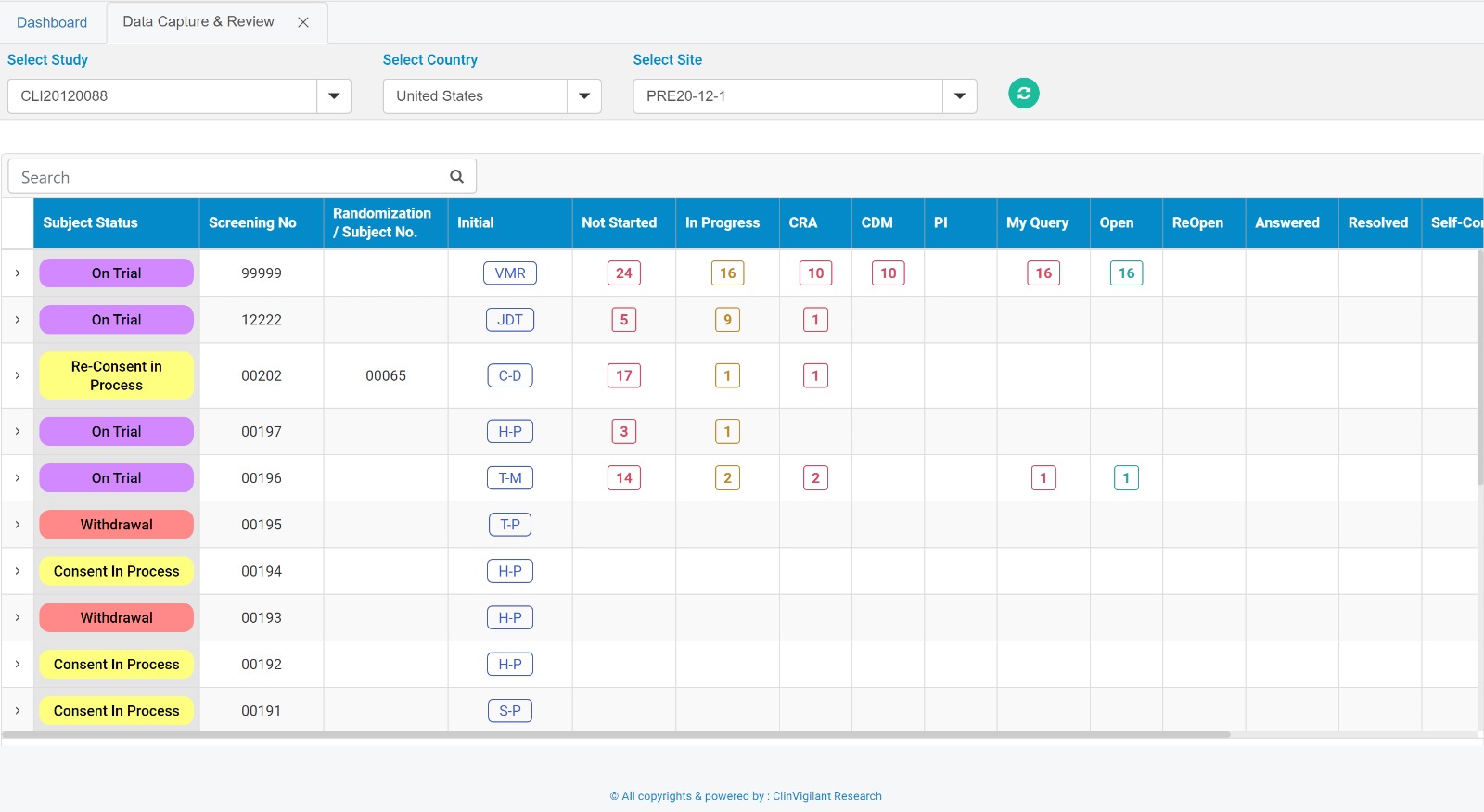
Clinvigilant EDC is designed to provide a wide range of configurable features, including the seamless creation of electronic Case Report Forms (eCRFs) from paper CRFs. No programming required, user friendly and cloud based.
Why ClinVigilant ?
Anyone can use our eclinical solutions to build a study in hours. Clinvigilant´s clients love our platform for three main reasons: its high configurability, user-friendliness, and cost-effective prices.
Proven 15+ Industry Experience
Regulatory
Compliant
User Friendly
and Easy to use
Cost
Effective
Secure Cloud
based platform
In house subject
matter experts